
I have always been fascinated by the intersection of structure and function — how building the right molecule can solve a complex problem.
Currently, I am a Senior Lecturer in Inorganic Chemistry at Manchester Metropolitan University (MMU). My research has evolved to combine my background in supramolecular chemistry with molecular electronics. My goal? To develop novel sensing platforms capable of detecting specific chemical species with high precision.
Beyond the lab, I am deeply committed to education. I teach within the Joint Educational Institute with Hubei University, where I deliver modules on Inorganic Chemistry and Laboratory Techniques. This role challenges me to communicate complex scientific concepts across cultural boundaries, ensuring the next generation of chemists is equipped with both theoretical knowledge and practical hands-on skills.
With over a decade of experience in multi-step synthesis, air-sensitive chemistry, and crystallography, I still love the technical side of the work. Whether it’s troubleshooting a reaction or designing a new curriculum, I enjoy the challenge of finding the solution.
Current Work
Latest Publication
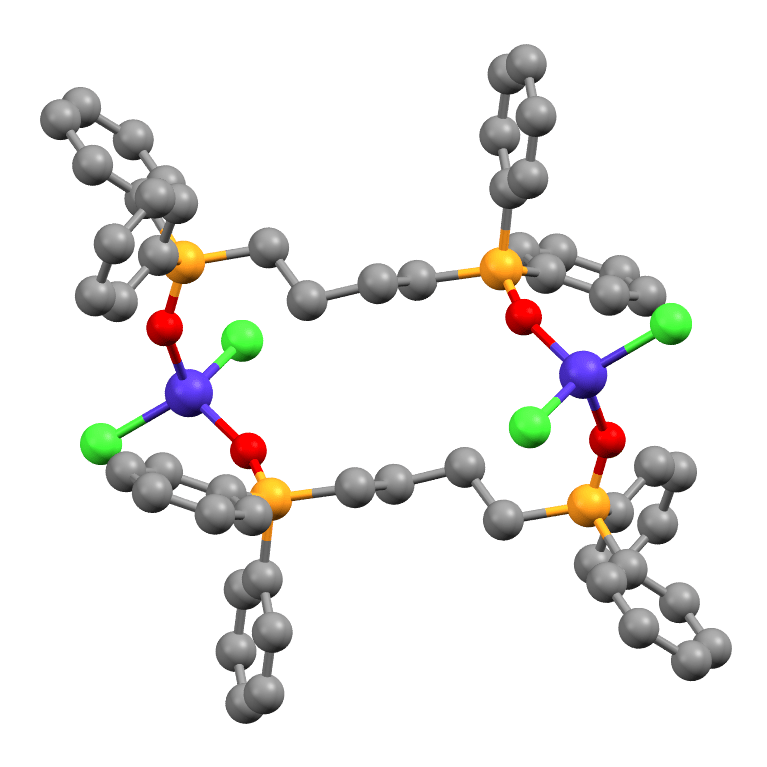
Robust Y and Lu TrenSal Catalysts for Ring-Opening Polymerisation
Eszter Fazekas, Alvaro Etcheverry-Berrios, Gary S. Nichols, Stergios Piligkos, Euan K. Brechin, and Jennifer A. Garden.
Abstract
Mono and bimetallic cage-like TrenSal complexes based on rare-earth metals Y and Lu were synthesised and fully characterised by single crystal and powder X-ray diffraction, multinuclear NMR and IR spectroscopy, and MALDI-ToF mass spectrometry. These robust and air-stable complexes are active catalysts for the ring-opening polymerisation of rac-lactide. While the polymerisation activity is low, the complexes remain active in air using unpurified monomers, and are highly unusual examples of metal-organic cages applied in polymerisation. MALDI-ToF mass spectrometry studies of the obtained polymers revealed H and OBn end-capped chains suggesting that an ‘activated monomer’ mechanism predominates when BnOH is used as an exogeneous initiator.
Polyhedron, 2024, 266, 117308. DOI: 10.1016/j.poly.2024.117308






You must be logged in to post a comment.